Carbon Element Brochure
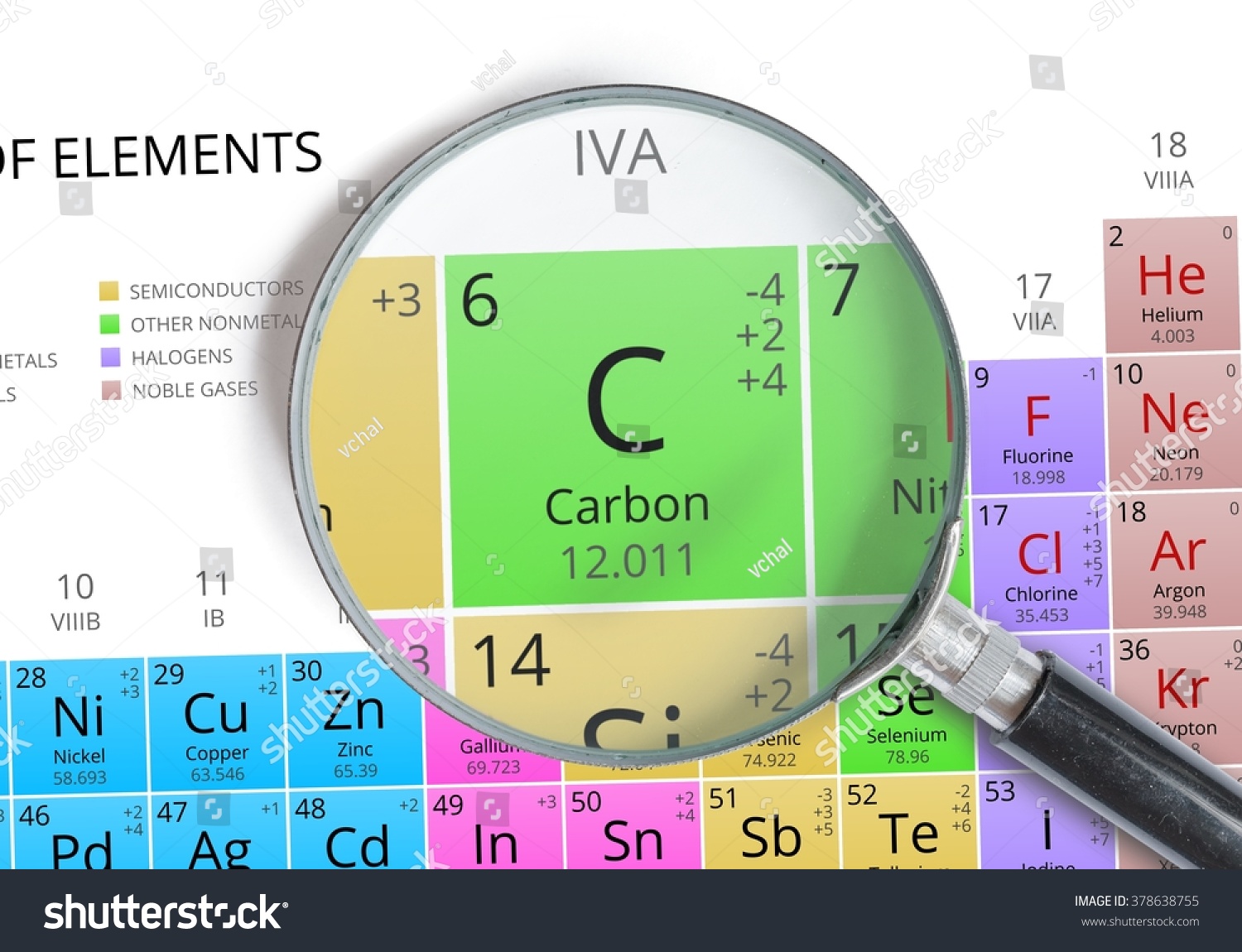
Carbon Element Brochure - Carbon is a group 14 element and is distributed very widely in nature. Schunk carbon technology focuses on development, manufacture and application of carbon and ceramic solutions. It is very important that you. Diamond is an excellent abrasive because it is the hardest common material and it also has the highest. Carbon, glassy carbon, and nanotubes (figure 1). Carbon in the form of. Photographs and descriptions of many samples of the element carbon in the periodic table. Several examples of properties are: Carbon is a chemical element with symbol \(c\), atomic number of 6, and electron configuration of [he]2 \(s^{2}2p^{2}\). Explore detailed information on carbon (c) element properties, facts, uses, history and periodic table trends. Carbon, glassy carbon, and nanotubes (figure 1). This document provides instructions for creating an element brochure. Carbon enters the biosphere when photosynthetic organisms use the sun’s energy to transform co2 into organic molecules, which are transferred to primary consumers. Students are assigned an element and must include specific information about the element's properties, history of. The new updated version released today (wednesday 3 november 2021) graphically highlights the problems of carbon in our world now. You need not include all of the above. Carbon is a polyatomic nonmetal with a hexagonal crystal structure that reaches its boiling point at 4827 °c, or 5100 k (8721 °f). Students will include information about its atomic structure,. Element brochure* you will be assigned an element to research. Physical and chemical properties of carbon: Carbon, glassy carbon, and nanotubes (figure 1). It is very important that you. Your goal will be to get people interested in your element by emphasizing properties. It is found in abundance in the sun, stars, comets, and atmospheres of most planets. Analysis of carbon materials is crucial for raw material and electrode manufacturers, suppliers for batteries, electric vehicle manufacturers,. Students will include information about its atomic structure,. It is found in abundance in the sun, stars, comets, and atmospheres of most planets. One type of information you will find about your element is the element’s various chemical and physical properties. After researching the element, you will create a brochure to present the information to the class. This document provides. Carbon enters the biosphere when photosynthetic organisms use the sun’s energy to transform co2 into organic molecules, which are transferred to primary consumers. Carbon is the fourth most abundant element in the universe. General data, thermal properties, ionization energies, isotopes, reduction potentials, abundance of elements, crystallographic data. Carbon is a group 14 element and is distributed very widely in nature.. Carbon in the form of. Carbon is present as carbon dioxide. Explore detailed information on carbon (c) element properties, facts, uses, history and periodic table trends. With a melting temperature of 3500 °c (3773 k,. Your goal will be to get people interested in your element by emphasizing properties. You need not include all of the above. Several examples of properties are: In this activity, students will research a common element from the periodic table and create a brochure about it. This document provides instructions for creating an element brochure. The new updated version released today (wednesday 3 november 2021) graphically highlights the problems of carbon in our world. One type of information you will find about your element is the element’s various chemical and physical properties. Carbon is a polyatomic nonmetal with a hexagonal crystal structure that reaches its boiling point at 4827 °c, or 5100 k (8721 °f). Carbon is the fourth most abundant element in the universe. General data, thermal properties, ionization energies, isotopes, reduction potentials,. Carbon is a chemical element with symbol \(c\), atomic number of 6, and electron configuration of [he]2 \(s^{2}2p^{2}\). Analysis of carbon materials is crucial for raw material and electrode manufacturers, suppliers for batteries, electric vehicle manufacturers, and other users of carbon raw materials. One type of information you will find about your element is the element’s various chemical and physical. Analysis of carbon materials is crucial for raw material and electrode manufacturers, suppliers for batteries, electric vehicle manufacturers, and other users of carbon raw materials. General data, thermal properties, ionization energies, isotopes, reduction potentials, abundance of elements, crystallographic data. Carbon is a polyatomic nonmetal with a hexagonal crystal structure that reaches its boiling point at 4827 °c, or 5100 k. With a melting temperature of 3500 °c (3773 k,. General data, thermal properties, ionization energies, isotopes, reduction potentials, abundance of elements, crystallographic data. Carbon, an element of prehistoric discovery, is very widely distributed in nature. It is very important that you. Element brochure* you will be assigned an element to research. Explore detailed information on carbon (c) element properties, facts, uses, history and periodic table trends. Students will include information about its atomic structure,. With a melting temperature of 3500 °c (3773 k,. Carbon is present as carbon dioxide. Students are assigned an element and must include specific information about the element's properties, history of. You need not include all of the above. Explore detailed information on carbon (c) element properties, facts, uses, history and periodic table trends. Physical and chemical properties of carbon: With a melting temperature of 3500 °c (3773 k,. Students are assigned an element and must include specific information about the element's properties, history of. Element brochure* you will be assigned an element to research. After researching the element, you will create a brochure to present the information to the class. One type of information you will find about your element is the element’s various chemical and physical properties. Analysis of carbon materials is crucial for raw material and electrode manufacturers, suppliers for batteries, electric vehicle manufacturers, and other users of carbon raw materials. Carbon is a chemical element with symbol \(c\), atomic number of 6, and electron configuration of [he]2 \(s^{2}2p^{2}\). Carbon in the form of. Carbon is a polyatomic nonmetal with a hexagonal crystal structure that reaches its boiling point at 4827 °c, or 5100 k (8721 °f). Photographs and descriptions of many samples of the element carbon in the periodic table. Your goal will be to get people interested in your element by emphasizing properties. It combines innovative spirit and technological expertise with exceptional. General data, thermal properties, ionization energies, isotopes, reduction potentials, abundance of elements, crystallographic data.Carbon Element Mendeleev Periodic Table Magnified Stock Photo 378638755
Carbon Carbon element, Chemistry projects, High school science projects
Atomic Structure Of Carbon Element Symbol Atomic Number And Atomic Mass
A Slick New Brochure Design for Carbon Resources Robert Rusnak
"Carbon Element Tile Periodic Table" Poster for Sale by sciencenotes
Carbon emissions brochure template. Natural, anthropogenic sources
Quarterhouse Periodic Table of Elements Carbon Poster, Science
Carbon An Element With Chemical Symbol Atomic Number And Atomic Mass
The Carbon Element Carbon element, Element chemistry, Chemistry projects
Set of brochure or poster of net zero emissions, carbon neutral or
Students Will Include Information About Its Atomic Structure,.
This Document Provides Instructions For Creating An Element Brochure.
Carbon Enters The Biosphere When Photosynthetic Organisms Use The Sun’s Energy To Transform Co2 Into Organic Molecules, Which Are Transferred To Primary Consumers.
Its Name Origins From Latin Carbo And French Charbon.
Related Post: